CASE STUDY – Novel Disease Detection Instrument
A startup company was developing a novel system for early detection of Alzheimer’s disease. The potential benefits of this system are far reaching. Early detection of this devastating disease will assist researchers in their efforts to find a treatment, as well as enabling large scale screening that will indicate the need to begin treatment, potentially preventing the onset of debilitating symptoms.
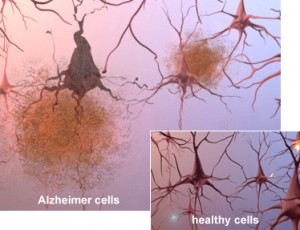
The instrument consists of a disparate collection of biological, electronic, mechanical, and optical subsystems. Coordinating these systems is embedded software running on ARM based micro-controllers, combined with a Windows Embedded Standard based user interface to be controlled by a Doctor or Clinician performing the test on a patient.
The innovative small company creating this system approached Lextel asking for help completing the development of the instrument, so that they could commence a clinical trial. Their development effort was in process, however, several key development engineers had decided to leave the company with the system unfinished and not working. The project was behind schedule.
Initial Analysis
The first order of business in this situation was to determine the current state of the system. This involved discussions with the remaining company team, review of written documentation, and hands on work with the system as it existed
Following the initial analysis, we developed a comprehensive list of shortcomings and needed enhancements to prepare the system for a clinical trial. This list included items that we could identify at the time of the analysis, however, as the project progressed, many more issues were discovered. These issues included both software issues that we could solve ourselves, as well as issues with various hardware and software components of the overall system that were being purchased from 3rd party vendors.
Instrument Development
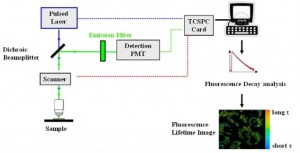
Over a period of approximately 6 months, we worked down the expanding list of outstanding issues. Some of this included re-write and completion of embedded software running on micro-controllers to complete the instrument functionality, ensure instrument safety, and insure reliability. The functionality required to run the diagnostic test on the human patient included control over linear motors, control and monitoring of a laser light source, control of florescence scanning hardware, result reduction and reporting, and internal diagnostics.
The user interface also needed significant work. This was running on a Windows CE, and later, Windows 7 Embedded Standard based embedded PC with operator interaction through a touch screen. In addition to completing the basic patient test functionality, the windows work entailed various support functions in the instrument, diagnostics, interaction with the various system components, and coordination with the embedded micro-controllers to effect the patient measurement.
The software development effort utilized engineers working from geographically dispersed locations. Coordination of the effort was one of the tasks that demanded special attention. The overall project planning for the clinical trial entailed the coming together of many diverse efforts. Delivery of the functioning instrument was but one these efforts. In the end, the instrument was delivered in a timely fashion in order to commence the clinical trial. Ultimately, the customer was successful in proving the safety and efficacy of the device in the clinical trial.
Broad Engineering Background
This example project illustrates the benefits of a broad based engineering background brought to bear on a problem. Because Lextel engineers have a background in hardware and software development, we were able to rapidly identify root causes of problems in the system. This included diagnosis of 3rd party vendor equipment and devices. This background is essential in order to avoid time wasting ‘finger-pointing’ situations when components from multiple, geographically dispersed vendors are brought together, but may not be working as expected.
A broad engineering background enabled us to identify problem root causes, and to be creative in coming up with solutions. Ultimately, these skills and capabilities are what allowed this project to be completed in a reasonable time frame.
Medical Instrument Tasks Completed
- Initial Analysis of Required Work
- Development of Embedded Control Software – ARM based micro-controller(s)
- Development of User Interface and Control Software – Windows XP Embedded and Windows 7 Embedded Stanard based
- Diagnosis and updates to FPGA based control logic
- Diagnosis and updates to electronic hardware modules
- Integration and Debug of 3rd party Linear motor control card and software
- Integration and Debug of 3rd party Fluorescence Lifetime Image Microscopy Hardware/Software
- Integration and Debug of Laser power monitoring hardware/software
- Oversight of remotely located engineers working on the project
- Ongoing oversight of project scheduling, prioritization
- Assistance with specification updates for FDA submission
- Delivery of completed instrument for Clinical Trial
Background Information on this project
Please let us know how we can help you!
